Think of the space each letter occupies as a specific, independent "set" on the sheet. The meaning of our message devolves from the relationship among a sheet's sets. We can even define "meaning" from the organization--the anatomy--of the sheet: What's in each set? How do the sets interrelate? The specific answers define the specific message. (I used to believe the genes for organ development, in principle, came on in this manner.)
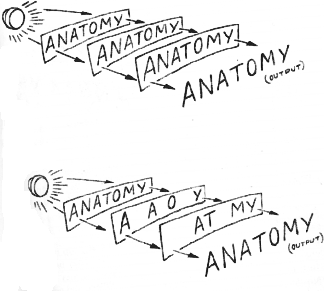
How do we simulate the brain with our ANATOMY sheets? More specifically, what can we do to model structural order among the several redundant sheets? The simplest way is to stack all sheets in exact correspondence or, as the printer says, perfect register.
Next, we want to observe our system--look at its output or readout, as information theorists call the display. The output, our analog of behavior, is what we see on the surface. With the sheets of the stack in perfect register, the output obviously is ANATOMY. But how many messages do we have in the system? We already know the answer for our imaginary system. But the real brain is an unknown.
What happens if we remove the first sheet from our model? Obviously, we still read ANATOMY at the surface; a little lighter, perhaps, but with its meaning quite intact. Does it make any difference which sheet we remove? Obviously not, as long as we keep the register constant. Suppose we erase or snip off the "TOMY" on the first sheet? Suppose we take "ANA" away from the bottom and "TOMY" away from the second? Our deletions may introduce subtle variations in intensity, but the meaning of our message survives as long as we maintain the equivalence of a sheet's worth of ANATOMY. Deleting, we can only destroy meaning at the surface if we clean out every last well of a particular sheet.

Our experiments model Lashley's experiments and lead to his basic conclusions. The important consideration is not where we erase or cut but how much. And there is nothing equipotential about our system. (The set on the left certainly isn't the same as that on the right!) These imaginary experiments served as my intellectual justification for rejecting Lashley's conclusions long before the hologram came along. They illustrate a set-theory model of gene activation I believed would account for organ regeneration--simultaneously, for memory as well as.
In designing an adequate test of hologramic theory, it is useful to ask why we were able to simulate Lashley's findings. The major reasons are these: First, our message is redundant. Second, we can preserve the analog of structural order by keeping the stack in register. Third, by subtracting or erasing, we can create the equivalents of empty or blank sets. We did nothing to the carriers of meaning, or to the free access surviving sets had to the readout. If our system had been an unknown, our subtraction experiments would merely tell us of its redundancy, and this property of the brain's information has been recognized since ancient times.
Suppose that instead of erasing sets, we rotate one of our sheets 180 degrees. Obviously, this manipulation will change the quality of our readout. Or suppose instead we switch the Y with the T? And what would happen if we simply gave the stack a shove and knocked the sheets out of register? The moment we alter the carriers of meaning, the system's anatomy, we distort the message.
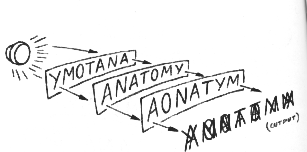
But in a hologram, the carrier of meaning--or phase-- cannot be reached with an eraser or a knife. Unlike our sheets, the hologramic code ought to survive any anatomical changes we can make. Herein is hologramic theory's most astonishing prediction: shuffling the brain will not scramble the mind! And how might we shuffle a living brain? The answer is embodied in the salamander.
***
Salamanders are amphibians, taxonomically one step up from fishes and a half-step down from frogs and toads. They begin life as aquatic creatures, and, a few species excepted, they undergo metamorphosis, become land-dwelling animals, and only return to the water to make new salamanders. (In the lab, I've seen adult salamanders them go into water after food; but they have to get out or they'll drown.)
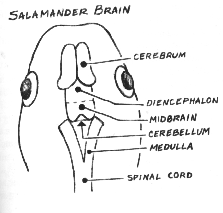
As larvae--the equivalents of toad and frog tadpoles, and before metamorphosis--salamanders of the genus I work with range down in size from the proportions of a six-year-old child's index finger, their digits barely discernible to the naked eye. Under a stereoscopic dissecting microscope, you can see blood corpuscles racing, scarlet, through vessels beneath transparent tissues, turning crimson as they reach the brushlike external gills on either side of the head. The gills, which they lose at metamorphosis, take oxygen from, and give carbon dioxide to, the clear spring-water environment. The larva does have lungs, but they are functionless little cellophane-like bags until the animal gets ready to move onto land. The larva's coloration recapitulates the basins and banks of its native woodland waters in the sunlight of an early spring morning: a thousand continuous hues of soft yellows; browns that range from almost-black through Dutch chocolate to the shades on the belly of a fawn; silver, in spots and patches, glinting like a slick of frost or a drop of dew. The larva's brain, textured like lightly polished Carrara marble and narrower than the letters on this page, can slip through the eye of a needle. Nevertheless, the tiny brain has the same major anatomical subdivisions as our own: cerebrum, diencephalon, midbrain, cerebellum, medulla. And within those minuscule neuroanatomical entities, somewhere, lie the programs for a range of complex, if primitive, behaviors.
Aside from flight, the most conspicuous manifestation of a salamander larva's mind is the quest for food. The salamander is a carnivore, but it is programmed to eat only living organisms. If it is hungry--and it usually is--the salamander will attack and devour whatever moves and can fit between its jaws. Indeed, it is to a crimson, threadlike tubifex worm, or a dainty daphnia, what a hungry wolf is to a careless pack rat or a stray lamb: imminent death.
Salamanders are born with the instinct to hunt. As embryos, yellow-white yolk stuffs their stomachs and intestines. Traces of yolk persist into the early larval periods; but within hours after the last molecules of the yolk have been consumed, thus clearing the alimentary canal for a meal, the larva is ready to strike at any prospective quarry. Although feeding is instinctive, behavioral nuances may be imprinted during early encounters with prey. The greater the struggle at the stage when feeding begins, the more finesse the larva seems to show in subsequent attacks. But animals deprived of early attack experiences still hunt later on, only with less elegance and efficiency.[1]
The active nature of the salamander's feeding is apparent in its movements in relation to different kinds of prey. When it is preying on water fleas, daphnia, or brine-shrimp embryos--any creature that jets through the water in jerky little spurts--the salamander larva waits motionlessly, poised until the victim comes close to its snout. Then, snapo! The larva's move is quick, sudden, accurate and deadly. Then it settles down to wait for more!
But with wriggling worms, such as tubifex or their cousins, the milk-white encyhtreas, the salamander's response is quite different. When it first senses a worm, it freezes, hangs suspended in the water like benign debris, or stands stock still at the bottom of the bowl. Moments may pass as it seems to be fixing the location of the worm. Then slowly it turns, usually pausing again, perhaps to recheck the azimuth of the prospective course. Half-walking, half-swimming, it now glides cautiously toward the fated worm. At its destination, it usually pauses once more and carefully, deliberately, moves its head back, forth and around the undulating mass, as though computing the tensor algebra of the quarry's ever-varying geometry.
I have seen this sizing-up stage last thirty or forty seconds, a seeming eternity in the waiting game. The salamander takes one last momentary pause. Then comes the strike. And into it, the salamander throws every bit of its might. The attack is not the "blip," as with brine shrimp or daphnia but a ferocious, violent assault that would knock down a building, if the salamander were the size of a great white shark. It locks the worm in its jaws and hangs on, no matter what escape maneuver the squirming victim tries; and it swallows the worm, live, bit by bit. If the worm is relatively short, it goes down quickly. If not, the process may take minutes, the salamander holding on with such tenacity that it can actually be lifted from the water by the free end of the catch.
Salamander larvae sense their prey in three known ways: sight, touch and sonar--shock-wave detection-- via what is called the lateral line system. The larva has the anatomy for smell, but by all indications it doesn't use this sense until close to metamorphosis into a land animal. It can use its other senses either singly or in any combinations, but it has a very strong predilection for the eyes, when it has them. For example, an animal with only one eye will turn, twist and aim the eye for a good look at a worm before making a strike. Yet an eyeless salamander can use either sonar or touch, or both, to launch a completely successful attack.
The salamander's capacity to endure massive injury has fascinated biologists ever since 1768, when Lazzaro Spallanzani published the fact that salamanders regenerate lost appendages. In larvae, a fully functioning replica replaces an amputated leg or tail in a month to six weeks; in adults, the process may take a year or more. Regeneration occurs again and again following subsequent amputations, as Spallanzani, no less, reported. Other organs and tissues regenerate as well. A severed optic nerve, for example, reestablishes contact with the brain, and the eye eventually can see again. In fact, almost all the salamander's nerve fibers grow back. Following an incision into the brain, new nerve fibers quickly sprout and soon reknit a completely functional patch across the rift.
Larval salamanders are also excellent recipients of tissue and organ transplants. They do possess tissue-rejecting mechanisms, but rejection is very sluggish and inefficient and often fails completely, the foreign graft becoming a permanent part of the host animal.
The larva's small size necessitates the use of a stereoscopic dissecting microscope for operations. With experience, the surgery becomes routine and requires only a few inexpensive tools. An anesthetic (MS 222), dissolved in the water, renders the animal unconscious.[2] A cream-colored Vermont marble clay lines the bottom of the operating dish, the clay molded to complement the contours of a salamander's belly. Ordinary straight pins, plunged into the clay and then crossed, truss the animal into the desired position. An ordinary needle with a honed point becomes the scalpel. But I use iridectomy (iris) scissors for most of the cutting, and "Genuine du Mont et fils" Swiss watchmakers' forceps for grasping, manipulating and blunt-dissecting.
The larva's skull cap consists of two microscopically thin translucent membranes, the forerunners of bony plates. The membranes meet at a midline seam, or raphé and a quick, upward, longitudinal stroke with the tips of the iridectomy scissors quickly separates them, exposing the brain. Even after the skull ossifies into bone, the opening up of the cranium requires no more force than does clipping a fingernail. Hemorrhage is not serious, and no problem at all with larvae. Working under the dissecting microscope, the operator can see and avoid the tiniest blood vessels. When the experiment makes cutting a vessel necessary, clots rapidly form and quickly plug the leaks. Unlike the organs of higher animals, those of the salamander can survive for days without a blood supply, particularly in a cool environment. In fact, the animal may be bled entirely and yet remain alive for a time. (In the early 1950s, the embryologist, Meryl Rose actually raised a colony of bloodless tadpoles.) The chilled animal's slowed metabolism reduces its demand for oxygen. After surgery, the thin skin lets sufficient oxygen pass in from the water to keep a transplanted organ alive until the host's vasculature hooks into it.
For operations on larvae, sutures are unnecessary. (Adult skin may require stitches, however.) But even with larvae, it is essential to cover a wound with skin. When gently pressed together, the cut edges of the skin adhere and send battalions of epithelial cells over the rift. Within days, the line of incision has disappeared completely.
The salamander larva was an excellent candidate for shufflebrain experiments on an additional count. After the healing period, messages would relay freely between the spinal cord and the shuffled brain. How did I know this? Some of my confidence grew out of an extensive series of preliminary experiments I will mention briefly in a moment. But as a student trying to teach myself the art of transplanting tiny organs, I assigned myself exercises involving the larval salamander's brain. First, I would make a tunnel in the Jell-O-like connective tissue of the dorsal fin. Then I would remove the brain, and store it in the tunnel. The animal, of course, went into a stupor. How did I know whether the brain had survived (whether I had passed or flunked my test)? After some days, I would return the brain to the cranium. Most animals survived. And in eight to twenty days, they recovered consciousness.
Preliminary to shufflebrain experiments, I conducted a systematic investigation of the salamander larva's medulla, the transition zone between the rest of the brain and the spinal cord. When I destroyed the medulla, the animals became unconscious and died within two weeks. If I left the medulla intact and amputated the brain immediately in front of it, the animals went into permanent stupor but remained alive for many months.

Here's a section through the head of an essentially brainless salamander larva, an example of "blanking." The large circular objects toward the edges of the photo are eyes. The zone between the eyes would ordinarily be filled with brain. Here we see it packed with connective tissue cells that had migrated in from elsewhere. This animal lived for many months after its brain had been amputated; it was behaviorally inert during the entire period. (Brainless animals do not undergo metamorphosis, incidentally, because they don't have the pituitary gland, one lobe of which makes the hormones that drive the process.)
My first formal plunge into shufflebrain experiments involved a repetition of Lashley's basic operation. I mapped the brain into regions. Then, in an extensive series of operations, I replaced given map regions with pieces of spinal cord, systematically moving down the brain, region by region, among the various subgroups of experimental animals.
The animals invariably fed the moment they recovered from postoperative stupor, no matter which region I removed. Massive destruction of the brain reduced feeding but did not stop it. Clearly, there was no exclusive repository of feeding programs in any single part of the salamander's brain.
My next series of experiments involved interchanging right and left hemispheres. Feeding survived. Next, I tried rotating the cerebral hemispheres 180 degrees around their long axes so that up faced down and down faced up. I did this with each hemisphere separately and then with both simultaneously. Feeding survived. Next I turned the cerebral hemispheres around so that the front faced to the rear. Feeding survived.
I then moved down to the diencephalon, where the optic nerves enter the brain. I knew from my own observations and from the vast literature on the subject that disturbing the diencephalon might affect vision. But I was sure that touch and sonar from the lateral line system would enable the animal to sense the worm. My first experiments in this series involved removing the diencephalon and fusing the cerebral hemispheres directly onto the midbrain. As I had expected, surgery blinded my animals. They were also much less active than before. But when a worm came close, the salamander would attack and eat it, diencephalon or no!
In the next series of experiments with the diencephalon, I either rotated the part 180 degrees or reversed it front to back. These animals did recover vision. But often, when they struck (or seemed to strike) at a worm, they'd move in the wrong direction. At least this was how I interpreted their strange behavior toward worms, on the basis of eye rotation experiments performed by Roger Sperry in the 1940s. However, when I dimmed the lights and forced the salamanders to use sonar and touch, instead of vision, they were able to attack the worms. (In other words it wasn't the whack in the head--diencephalon to be more precise--that had induced the weird visual response.)
My next experiments involved the exchange of brain parts. For example, I switched the diencephalon with the cerebrum. I moved the midbrain up front and either the cerebrum or the diencephalon to the rear. I performed every operation I could think up. But nothing eradicated feeding. Let me describe one series of rather drastic operations in more detail.
I decided to see what effect an extra medulla would have on feeding. The best approach, I thought, would be to remove the entire brain of one animal, down to and including its medulla, and fuse it onto the medulla of another animal--the host. The available space inside my prospective host's cranium, however, wasn't sufficient to accommodate this amount of brain--not if I used a sibling as a donor (which I most frequently did). Therefore, I decided to use a large tiger salamander larva (Amblystoma tigrinum) as the host and a little marble salamander larva (Amblystoma opacum) as the donor. The fit was perfect, and the operations went very well. A week to ten days later, all five of the animals in the group were awake and feeding again. And no facet of their behavior even hinted at the bizarre contents of their crania.
I suspect that most experimentalists suffer, from time to time, from what I call janitor-induced paranoia--the certainty that in the middle of the night the janitor, or someone, has exchanged the labels on the test tubes, cages, or salamander dishes. I certainly suffered a bad case of the syndrome while observing the behavior of members of the tiger/marble salamander experiments. I repeated the operations and got identical results. But my disbelief simply would not go away. I mean, here was a brain in a foreign head, plugged into the medulla of a different species--a brain with two medullas, no less. And yet the beast behaved like a normal salamander. And every time I checked for vision (with mirror images of worms), the animal showed me it could see, which meant that the transplanted brain could process visual perception delivered by the foreign eyes of its host. One Sunday morning I finally gave up, pickled some of these animals, and dissected their brain cases. There was no question about it. Each had two medullas, one plugged into the other. And I could trace the host's optic nerves into the transplanted brain. Even if Lashley had been our janitor, I would have been forced to conclude that shuffling the brain had not scrambled feeding.
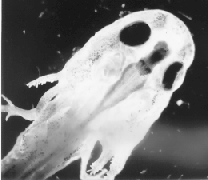
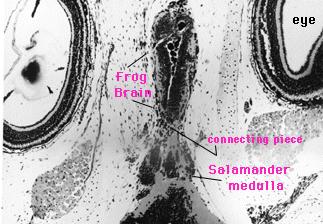
This is a dissection of a tiger salamander larve whose brain had been amputated forward of the medulla and replaced by the entire brain--including the medulla--of a smaller, marble salamander larva. The donor brain is the deep gray structure between the specimen's eyes; the host's medulla is the large whitish trough behind the donor medulla. The behavior of these animals was identical to that of the controls in all discernible respects.
Now the adult leopard frog is a notorious carnivore. Yet as a young tadpole it is a vegetarian. When the tadpole bothers a tubifex at all, it is only to suck algae and fungi from the worm's wriggling flanks.
I called the first member of this new series Punky. Punky the tadamander! He had the body of a salamander (Amblystoma punctatum), but the brain in front of his medulla had come into this world in the head of a Rana pipiens.
***
The most obvious question about Punky was whether or not a frog's brain would even connect, anatomically, with a salamander's medulla. But this question had to wait. To answer it for certain, I would have to kill Punky. Meanwhile, it was only my hunch that his new brain would reinstate overt behavior. If he ever came around, I'd actually have to observe him--with my own eyes--eat a worm in order to vindicate my hypothesis.
By seventeen days after surgery, Punky had fully regained his ability to stand and swim. Within a few days after that, he had become the liveliest animal in my colony. (Tadpoles are more active than salamanders.) He was blind, but his sonar and sense of touch were in splendid working order. When I dropped a pebble into his bowl, the "clink" would alert him and bring him swimming over immediately. And so would a newly presented tubifex worm.
But the worm was completely safe. Punky would inspect the squirming crimson thread for perhaps a minute or two. Then he would execute a crisp about-face! and swim away. And this continued for the next 68 days, while I virtually camped at the edge of his dish. A conscious, alert and responsive little fellow he remained throughout. But not once during that time did Punky even hint at an attack, despite the fact that a fresh worm was always available for the taking. Worms were now objects of lively curiosity, not of furious assault. If feeding programs existed in Punky's medulla, their presence would have to be accepted on the strength of divine revelation, not experimental fact. My last-ditch hypothesis failed the pragmatic test of truth: it didn't work.
I had performed an even dozen tadamander operations. None of the tadamanders fed, but eight must be discounted because, although they lived for months, they never regained consciousness. I also introduced a number of control operations into the study. In one control series, I evaluated variables of surgery by transplanting the same regions of the brain I had transplanted to produce the tadamander, but with salamanders as donors, instead of tadpoles. These animals regained typical salamander feeding behavior in less than three weeks. What about the possibility that tadpole tissue had some general inhibitory effect on salamander feeding? I controlled this by transplanting various tadpole tissues (brain or muscle or diced tadpole) to salamanders' tail fins or abdominal cavities. These salamanders exhibited no changes in feeding habits. But perhaps frog-brain tissue had an active inhibitory influence on salamander feeding behavior. To test this, I transplanted portions of tadpole brain in place of the salamander's cerebral hemispheres, leaving the rest of the host's brain intact. These animals ate the worms, right out of surgery. Because tadamanders would not eat spontaneously, I had to force-feed each one, which became a major undertaking. Twice a week, I lightly anesthetized each tadamander and inserted fresh salamander muscle into its stomach. Of course, I also had to control the feeding procedure. What if the meal satiated the animal or the anesthetic dulled its appetite? I anesthetized and force-fed normal animals, but neither the anesthetic nor the meal affected their appetites.
The extra work had made Punky the tadamander's season a long one. And I might have terminated the experiment much sooner if I had not grown fond of him.
One morning, I arrived in the lab to find all the active tadamanders except Punky displaying signs of something I had dreaded from the outset--transplant rejection! Fortunately, Punky was still healthy. But I doubted that he would last very long. And I could not risk losing the critical anatomical data inside his cranium.
I stained alternate slides of Punky's tissues in two different ways. One procedure, a widely used all-purpose method known as hematoxylin and eosin, enabled me to judge the overall health of the tissue at the time of preservation. The other method, called Bodian's protargol stain, involved depositing silver salts on very fine nerve fibers, fibers that otherwise do not show up under the microscope. Bodian's stain is tricky. And the moment the technician handed me the slides, I selected one at random merely to check quality. But that very slide had the answer I sought. A cablework of delicate nerve fibers connected the tadpole brain to the salamander medulla. It is irrational, I confess, but I date my belief in hologramic theory from that first look at Punky's brain.
***
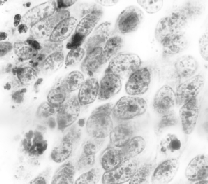
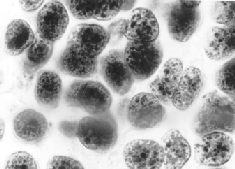
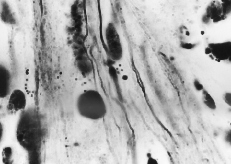
The larger oval and round entities are various nuclei. Many of the smaller, grain-like bodies are known as lipofucsin granules; they are indications in general (including human brains) of the onset of degeneration and, I subsequently found, herald brain-graft rejection in this kind of preparation. Not only did Punky's slides tell me that his transplant had established neurological connections with the host parts; they also indicated that I preserved him just in the nick of time. He would have died in a day or so. In fact, if I'd waited just 24 hours more, the nerve fibers might have degenerated beyond microscopic identification.
Internet contact:pietsch@indiana.edu